Edition_7
Edition_7
Today we will delve into the structure and composition of the various clay minerals:
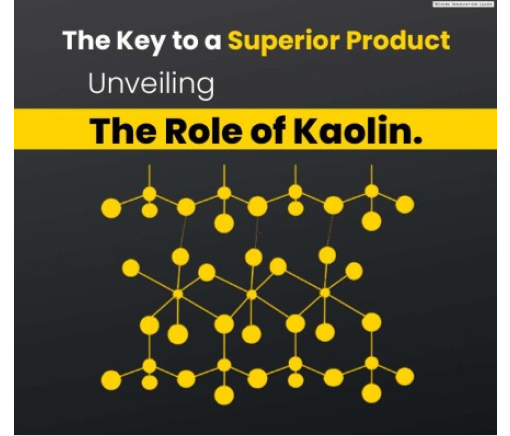
The atomic structure of the clay minerals consists of two basic units,
An octahedral sheet
A tetrahedral sheet.
The octahedral sheet is comprised of closely packed oxygens and hydroxyls in which aluminum, iron, and magnesium atoms are arranged in octahedral coordination
The second structural unit is the silica tetrahedral layer in which the silicon atom is equidistant from four oxygens or possibly hydroxls arranged in the form of a tetrahedron with the silicon atom in the center.
These tetrahedrons are arranged to form a hexagonal network repeated infinitely in two horizontal directions to form what is called the silica tetrahedral sheet

Classification of the clay minerals
I. Amorphous Allophane group
II. Crystalline
A.Two-layer type (sheet structures composed of units of one layer of silica tetrahedrons and one layer of alumina octahedrons)
1. Equidimensional Kaolinite group Kaolinite, dickite and nacrite
2. Elongate Halloysite
B.Three-layer types (sheet structures composed of two layers of silica tetrahedrons and one central dioctahedral or trioctahedral layer)
1. Expanding lattice
a. Equidimensional Smectite group Sodium montmorillonite, calcium montmorillonite, and beidellite Vermiculite
b. Elongate Smectite Nontronite, saponite, hectorite 2. Non-expanding lattice Illite group
C. Regular mixed-layer types (ordered stacking of alternate layers of different types) Chlorite group
D. Chain-structure types (hornblende-like chains of silica tetrahedrons linked together by octahedral groups of oxygens and hydroxyls containing Al and Mg atoms) Sepiolite Palygorskite (attapulgite)
Proposed by Grim in his book (1968), which is a basis for outlining the nomenclature and differences between the various clay minerals